Comparative of the main sources of potassium applied in fertirrigación for the agriculture
José Manuel Fontanilla, director of Marketing of
In agriculture there are three fundamental sources to contribute this essential element by means of the fertilisation, using nitrate potásico (KNO3), sulphate potásico (K2UNDER4) or chloride potásico (KCl). With the end to choose the best source of potassium for our crops go to explain the physical characteristics-chemical of each one of them (Table 1).
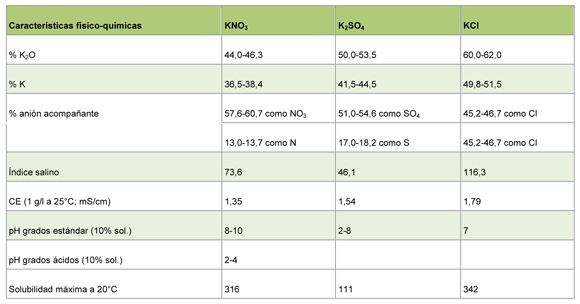
Table 1: physical Characteristics-chemical of nitrate of potassium, sulphate of potassium and chloride of potassium.
Nutritious value vegetable
The nitrate potásico contains 13% nitrogen (N) and 46% potassium (K2Or). Both are macronutrientes consumed by the plant entirely, not leaving waste of other elements that can be hurtful. The synergistic effect between K+ and NO3- facilitates the absorption of both ions by part of the roots of the plant, increasing the absorption of the same. Besides, the affinity between the nitrate loaded negatively and the potassium loaded positively avoids the absorption of this last to the particles of the floor, doing that it was available for the plants during a more prolonged time.
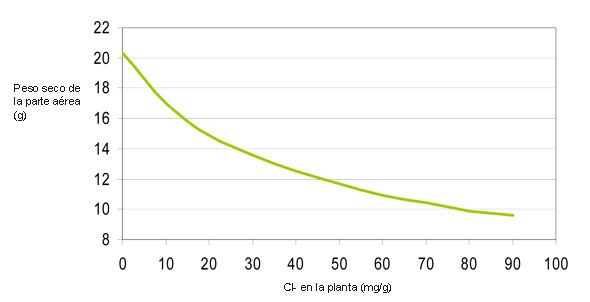
The sulphate potásico contains 50% of potassium (K2Or) and 54% of sulphate (UNDER4). The relation UNDER4/K2Or that find us in the plants is of 1:20, by what the fertilisation with sulphate potásico leaves considerable quantities of sulphate, in excess, in the solution of the floor. Chloride potásico contains 60% of potassium (K2Or) and 46% of chloride (Cl). The existence of chloride in the solution of the floor affects negatively to the plants. When it increases the concentration of chloride in the solution of the floor, the plants take said chloride instead of the nutrients aniónicos essential, especially the nitrate, affecting the development of the plant. When the quantities of chloride increase produce toxic effects, that can carry to the loss of performances and even to the death of the plant (figure 1).
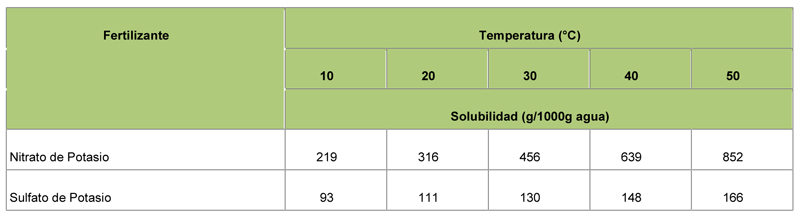
Solubility
Compatibility
Index salino and electrical conductivity
The Index Salino (IS) is a measure of the concentration of salts that induces a fertilizante in the solution of floor (Mortvedt, 2009). To greater index salino of the fertilizante, greater problems of salinity go to have with this.
The IS of a material expresseses like the relation of the increase of the pressesure osmótica produced by a fertilizante specific, with regard to the pressesure osmótica generated by the same weight of nitrate of sodium (NaNO3). The IS of the nitrate of sodium is the index of reference taking a relative value of 100. When it applies beside a seed, fertilizantes with low value of IS will be able to ensure a good germination without problems of proliferation. To equal weight, the nitrate potásico has a value of IS (73,6) minor that the chloride potásico (116,3) (table 1). In addition to IS, exists another indicator used to expresses the potential risk of the salinity in the floor, that is related with the effect of the solution fertilizante on the Electrical Conductivity (CE), to greater CE greater potential risk to produce symptoms by salinity in the crops.
The Electrical Conductivity (CE) of an electrolytic solution is the measure of his skill to drive the electricity. The Nitrate potásico has the lower value of CE (Table 1), compared with other sources of potassium.
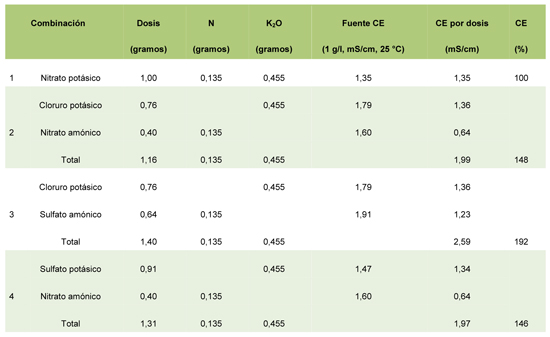
Table 3: Effects in the CE of several combinations of fertilizantes, compared with KNO3, keeping constant the levels of N and K.
Conclusions
The importance to contribute free potassium of chloride is very known, since the chlorine is an element that produces fitotoxicidad to the plants, by what the application of chloride potásico like fertilizante would have to limit to some crops tolerantes. Comparing the others two existent sources in the market of potassium the Nitrate potásico offers the following profits:
- Better alimentary composition for the plant.
- Better exert like source of K.
- High solubility and rapidity of dissolution.
- Wide rank of compatibility with other fertilizantes and agroquímicos.
- Does not interfere with the absorption of other ions by part of the plants.
- Minimum effect on the pH of the floor.
- Low contribution to salinity of floor.
Bibliographic references
- Mortvedt, J.J. 2009. Calculating salt index.
- Haifa group Website.
http://www.haifa-group.com/ - Potassium nitrate association website.
http://www.kno3.org/






